How Much Oxygen Is Present in 5 L of Air
Since at STP 1 mole of gas occupies 224 L simply divide 021224 to arrive at 00094 moles of oxygen. HowStuffWorks states that 5 percent of the volume of air is consumed each time a person inhales and is converted to carbon dioxide.
How To Calculate Fio2 From Liters Biomadam
This science projects allows you to find this percentage for yourself through examining a chemical reaction between oxygen and rust.

. Most of the time the air in the atmosphere contains the proper amount of oxygen for safe. N dfracVolVolSTP dfrac021224 00093 moles. Therefore 278 moles of molecular oxygen will occupy 6222 liters.
Each cell uses and requires oxygen to thrive. The human body takes the oxygen breathed in from the lungs and transports it to the other parts of the body via the bodys red blood cells. 21 mol O2 per 100 mol of air.
The answer is around twenty percent. The atmosphere is made up of a variety of gases including nitrogen oxygen argon carbon dioxide neon helium methane and so on. Air is a mixture of several gases where the two most dominant components in dry air are 21 vol oxygen and 78 vol nitrogen.
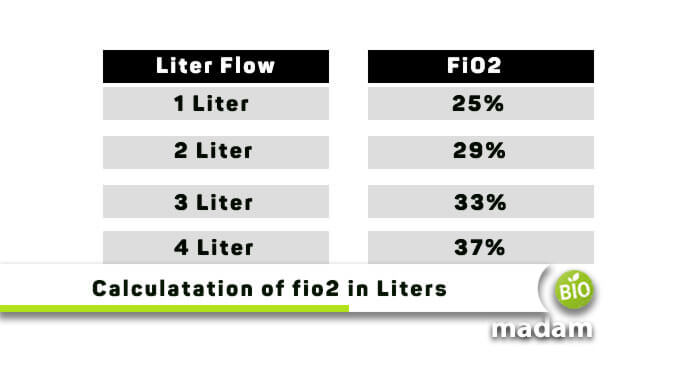
Nitrogen is the other major component of air 78. At 25 C and 1 standard atmosphere 1013 kPa of air freshwater can dissolve about 604 milliliters mL of oxygen per liter and seawater contains about 495 mL per liter. When the dial is set at 1 liter 24 percent oxygen is being delivered.
What volume of oxygen is present in 5 liters of air. The minimum oxygen concentration in the air required for human breathing is 195 percent. The oxygen content in systemic venous blood is 150 mlL and that for systemic arterial blood is.
Answer 1 of 5. No the density mass of a liter of air changes with temperature and pressure so you would have to define those. What property of nitrogen have you discovered as a result of this experiment.
At standard temperature and pressure 1 mole of a gas occupies 224 liters. If the flow meter is set at 2 liters the oxygen delivered goes up to 28 percent. Water has density of 1g cm cube so it means that 1litre water is 1000gram by formula densitymassvolume now we know that water isH2O One mole water is 2hydrogen1gram1Oxygen16gram18gram One mole water has 16 g oxygen and 2 g hydrogen- - - - - - - let this b.
How much oxygen is present in air. 21 Oxygen 78 Nitrogen 1 other gasses in out atmosphere. For each increase in the number on the flow meter dial the amount of oxygen delivered increases by 4 percent.
You can do your own test of how much oxygen is in the air by making a controlled environment and using a common material to pull the oxygen out of the air. If air is 21 molecular oxygen then we would need 6222021 or 2963 liters of air to provide that much molecular oxygen. Since both of these elements are diatomic in air - O2 and N2 the molar mass of oxygen gas is 32 gmol and the molar mass of nitrogen gas is 28 gmol.
Answer 1 of 3. About 1 litre - air is around 20-21 oxygen. The highest level of liters allowed is 6 and delivers 44 percent oxygen.
The concentration of water vapor a greenhouse gas varies significantly from around 10 ppm by mole fraction in the coldest portions of the atmosphere to as much as 5 by mole fraction in hot humid air masses and. Air is a mixture of several components like nitrogenoxygenCO2 and some small gases. How much oxygen is present in 5 liters L of air.
Oxygen has a molar mass of 159994 gmol and nitrogen has a molar mass of 140067 gmol. The Scripps Institute report oxygen measurements as changes in the O 2 N 2 ratio of air relative to a reference. At rest the average adult inhales around 20 percent oxygen in the air and exhales about 15 percent oxygen according to HowStuffWorks.
At rest typically 250 ml of oxygen is consumed 200 ml of carbon dioxide is produced and the cardiac output is 5 L all per minute. Air is a mixture of several components like nitrogenoxygenCO2 and some small gases. Since O2 has a moleculare weight of 32 and air 29 on a massmass basis this is 021 x 3229 0232 kg O2 kg air.
As the altitude of an area increases the density of. The solubility of oxygen in water is temperature-dependent and about twice as much 146 mgL dissolves at 0 C than at 20 C 76 mgL. It contains 78 of nitrogen and 21 of oxygen and other 1 is for smaller gases.
δ O 2 N 2 sample O 2 N 2 reference O 2 N 2 reference where O 2 N 2 sample is the O 2 N 2 mole ratio of an air sample and O 2 N 2 reference is the O 2 N 2 mole ratio of their reference. The primary ingredient is nitrogen followed by oxygen. The three major constituents of Earths atmosphere are nitrogen oxygen and argonWater vapor accounts for roughly 025 of the atmosphere by mass.
Show all of your work. The air is made up of about 21 percent oxygen. How many liters of gaseous oxygen will 1 liter of liquid oxygen produce.
How many moles of oxygen are in air. At standard conditions 1 liter of air at 21 oxygen possesses 021 L of oxygen. People inhale and exhale approximately 7 or 8 liters of air each minute or 11000 liters per day.
In normal air it is 21 O2 on a mole fraction basis or. Even though we sometimes assume that all air is oxygen only about a fifth of the atoms that make up our atmosphere are oxygen. -We all know that at STP conditions the volume of I mole of any gas 22414 L 22414 cm3 or ml So the volume of 1mole of oxygen 22414 L -We will now calculate the number of moles of oxygen in 1 L of air and containing 21 oxygen by volume.
How Much Oxygen Is Present In Exhaled Air By Volume Quora

What S In The Air Center For Science Education
What Is The Percentage Of Carbon Dioxide And Oxygen In Inhaled And Exhaled Air Quora
Comments
Post a Comment